Listeria monocytogenes Chromogenic Media
Detection & enumeration of Listeria monocytogenes
- Most complete range of solutions on the market
- Internationally validated methods
- Easy protocols (bag – plate)
Potřebujete více informací?
bioMerieux has developed a complete offer with 2 complementary culture media to perform all your Listeria monocytogenes analysis.
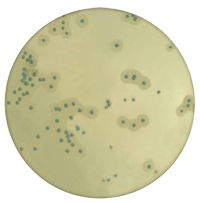
ALOA® : One media, many solutions
The ALOA® range offers a variety of validated methods to perform detection, enumeration and confirmation of Listeria monocytogenes.
ALOA® One Day enables detection of Listeria monocytogenes within 48 hours (enrichment included) in food and environmental samples
- One day results after enrichment for negative and confirmed positive samples
- Easy protocol: 1 bag – 1 plate
- Flexibility & workflow optimization: broth bags and plates can be held at +2°C to +8°C for up to 72 hours after incubation
- International validations (ISO 16140 / AOAC RI)
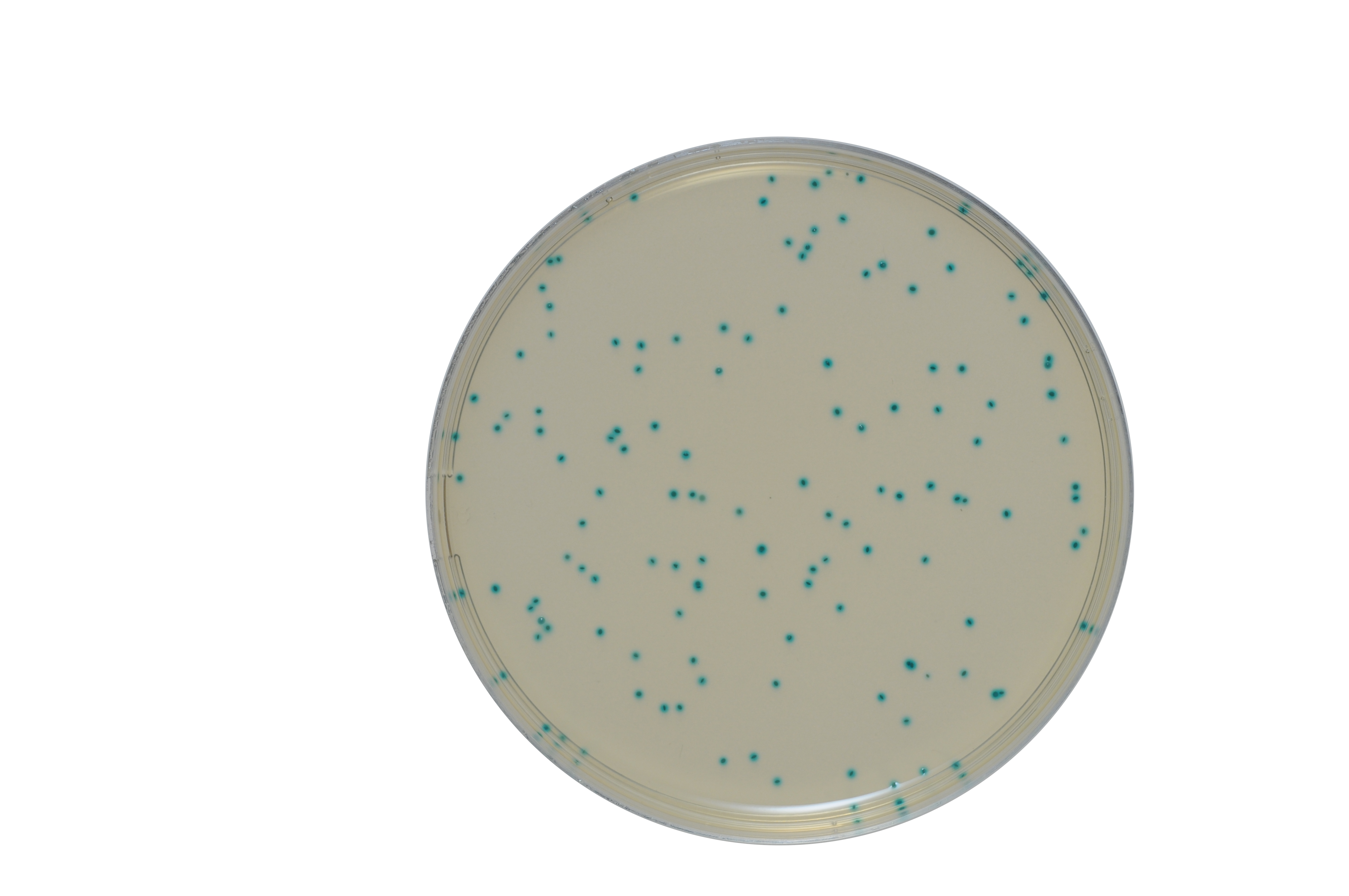
ALOA® Count method was designed for the enumeration of Listeria monocytogenes in food and environmental products
- Time to results 48 hours on ALOA plate
- Easy protocol: One plate per dilution
- Flexible and cost-effective:
- Save one hour: optional resuscitation step
- Optimize workflow: resuscitation in BPW or half fraser with antibiotics lets carry out detection and enumeration from the same suspension
- Plates can be held at +2°C to +8°C for up to 72 hours after incubation
- For low numbers < 10 CFU/g, several possibilities:
1 plate Ø90mm with pour plate method
1 plate Ø140mm with spreading method
3 plates Ø90 mm with spreading method
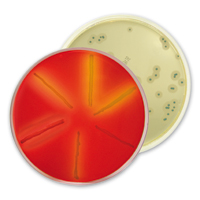
Confirmation methods
For the confirmation of these 2 methods, several validated solutions adapted to your release times:
- Same Day:
Fast Rhamnose: cost effective confirmation; positive from 6hrs; negative in 24 hrs
VIDAS® LMO2
Accuprobe® L.mono
- Next Day (24h):
ALOA® confirmation: Simple and cost-effective solution, Up to 6 confirmations per plate
API® Listeria: confirmation & identification
ALOA® is compliant with ISO 11290 reference methods (part 1 and 2) for detection and enumeration
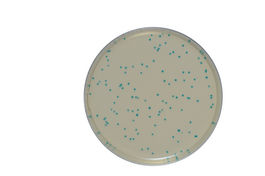
chromIDTM Lmono
Our chromID solution is designed for 2 specific applications:
- Detection of L.mono in highly L.spp contaminated samples
Short protocol (48h): one bag - one plate
ISO 16140 validated method
Easy-to-read thanks to a unique characteristic colour (blue colonies)
- Enumeration of L.mono within 24 hours
One-day result for confirmed L.mono enumeration
ISO 16140 validated method
Flexible & cost-effective:
- Plates can be held at +2°C to +8°C for up to 72 hours after incubation
- For low numbers < 10 CFU/g, several possibilities:
1 plate Ø90mm with pour plate method
2 plates Ø90 mm with spreading method
Other media for the detection and enumeration of Listeria monocytogenes
Among other media, our range includes the Half-Fraser broth, in different formats:
Bottles of 225 mL, Bags (3L, 5L), etc…
To discover our entire range of plates and bottled media for the detection and enumeration of listeria monocytogenes in food products, visit our e-catalogue.